VITANOVA BIOMEDICAL’S CANCER THERAPY TECHNOLOGY PLATFORM IS CALLED LIGHT-ACTIVATED INTRACELLULAR ACIDOSIS (LAIA)
Vitanova Biomedical (VNB) has developed a proprietary energy-based technique to elicit cancer-specific intracellular acidosis. The foundation of VNB’s core technology combines three proven principles accepted in today’s cancer therapeutics:
- Intracellular acidosis kills cancer cells
- Compounds can be activated by specific wavelengths of light.
- Nanoparticles can be actively targeted to only cancer using antibody/antigen conjugation
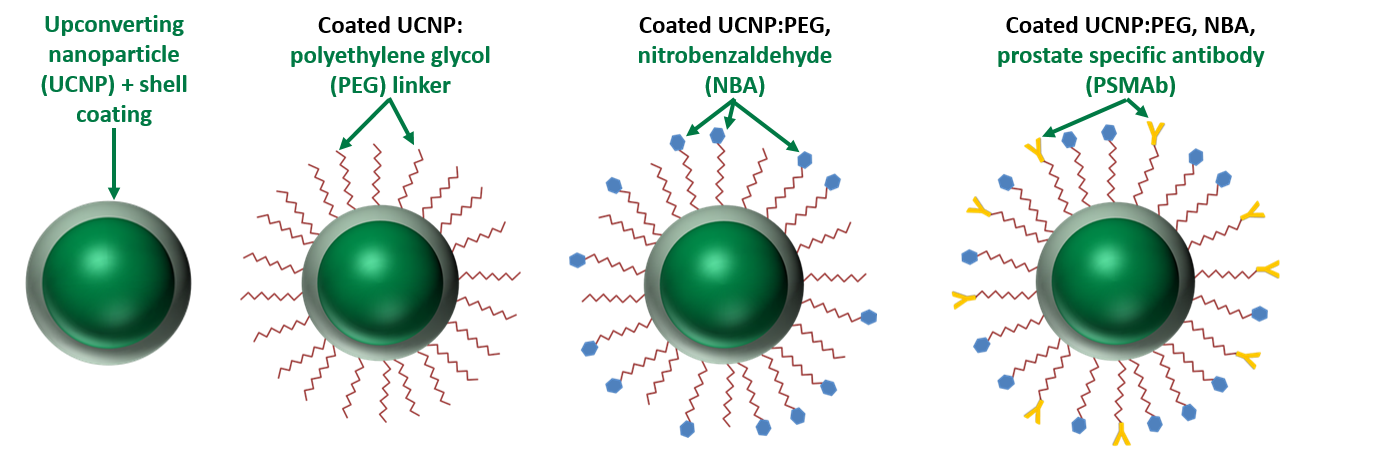
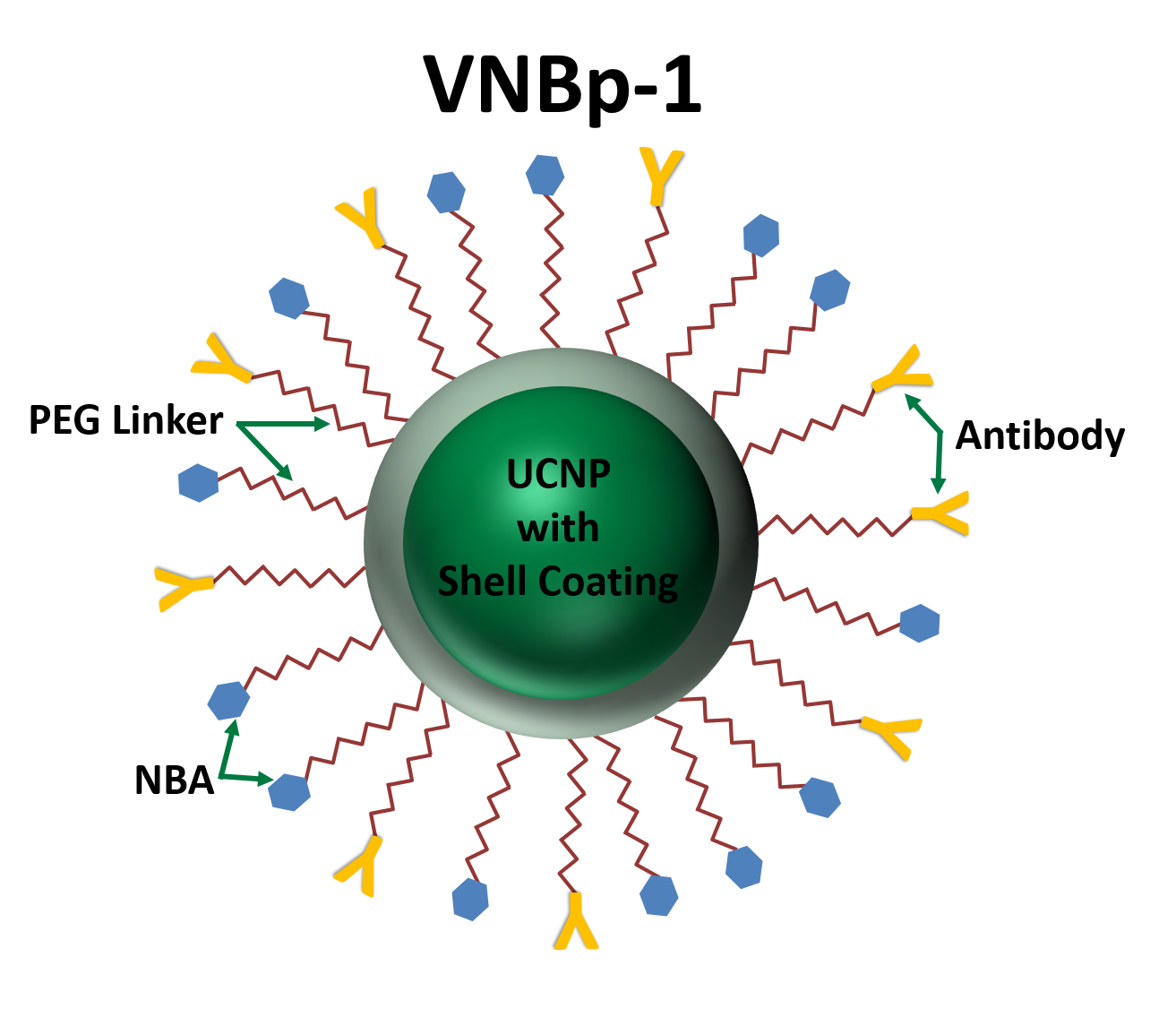
Rapid intracellular acidosis is achieved by the light-activation of VNB’s lead compound (VNBp-1), a yttrium (Yt) and thulium (Tm+) based upconverting nanoparticle (UCNP) conjugated to nitrobenzaldehyde (NBA), an acid donor that releases hydrogen ions in response to ultraviolet light. VNBp-1 is formulated to become photo-activated by deep-penetrating 980 nm near infrared light, which it then converts the 980 nm light to the ultraviolet light needed to release protons from NBA. VNBp-1 is also conjugated to prostate specific membrane antibody (PSMAb), which will selectively bind to prostate specific membrane antigen (PSMA) found only on the surface of prostate cancer cells. Once bound to the antigen, VNBp-1 will undergo endocytosis and become internalized in the prostate cancer cells.
 Currently, VNB is preparing to initiate the preclinical and IND-enabling studies utilizing their proprietary LAIA technology. As an initial therapy indication, the company plans to treat early-stage local prostate cancer for first line treatment with VNBp-1 in combination with a trademarked 980 nm light source and a trademarked endoscopic fiber optic cable.
Currently, VNB is preparing to initiate the preclinical and IND-enabling studies utilizing their proprietary LAIA technology. As an initial therapy indication, the company plans to treat early-stage local prostate cancer for first line treatment with VNBp-1 in combination with a trademarked 980 nm light source and a trademarked endoscopic fiber optic cable.
During this preparation to initiate the preclinical and IND-enabling studies, VNB is executing upon a focused five-step sequential development/testing process which includes:
- Demonstrating the effectiveness of LAIA in killing cancer utilizing multiple cancer cell lines in vitro
- Confirming the feasibility of utilizing LAIA to treat cancer in vivo
- Demonstrating the feasibility of utilizing an UCNP to deliver LAIA to cancer cells in vitro
- Formulating and optimizing the UCNP to actively target only prostate cancer in vitro
- Conducting Proof of Principle (POP) animal studies utilizing VNBp-1 in prostate cancer in vivo
The following information provides an overview of the results from the before mentioned steps 1-4, and high-level plans for conducting and completing step 5:
1. LAIA therapy (with NBA only) is effective in killing multiple cancer cell lines in vitro :
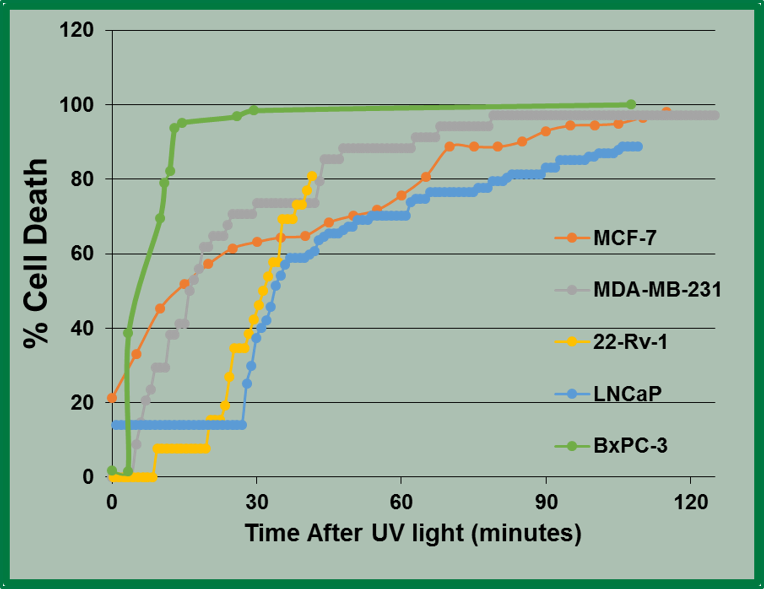
We first demonstrated that LAIA was effective in killing multiple cancer cell types. LAIA therapy produces a rapid and precise intracellular acidosis that cancer cells cannot overcome, causing them to enter the apoptosis pathway. This technology involves the photo activation of NBA, an acid donor, which releases H+ in response to ultraviolet light. In vitro data show that VNB’s LAIA technique is not cancer indication specific, as LAIA has successfully induced 81-100% cell death within 2 hours in two breast cancers (MCF-7), including the very aggressive triple negative breast cancer (MDA-MB-231), two prostate cancers (LNCaP and 22RV1), and a pancreatic cancer (BxPC3).
2. LAIA therapy (with NBA only) is a feasible treatment for cancer in vivo :
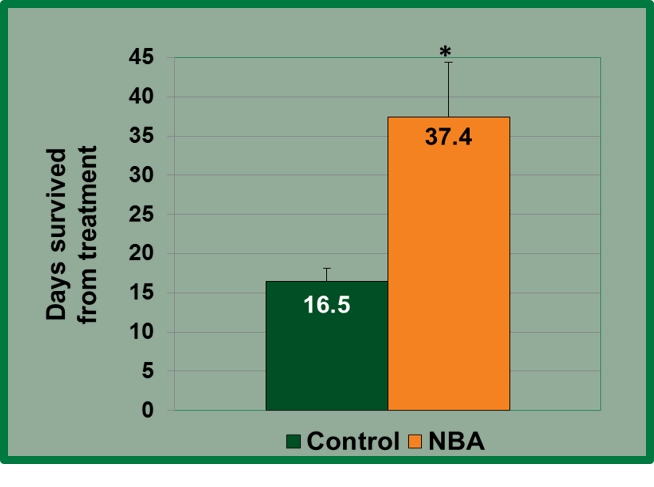
When injected intratumorally in nude mice bearing triple negative breast cancer tumors, NBA diffused across the tumor cell membrane and remained inside the cancer cells. Activation of NBA by ultraviolet light via an intratumoral fiber optic cannula caused a chemical conversion of NBA to produce a rapid and precise intracellular acidosis, resulting in significant reductions in triple negative breast cancer tumor growth rates and increased survival. In addition, there were no visible or behavioral side effects in the mice treated with NBA and light. Animal survival was significantly improved in the NBA treated mice (37.4 ± 7.0 days) compared to the control animals (16.5 ± 1.6 days) demonstrating the feasibility of photo activating NBA intratumorally to reduce tumor volume/growth rate in an animal model.
3. It is feasible to deliver LAIA to cancer cells utilizing an UCNP:
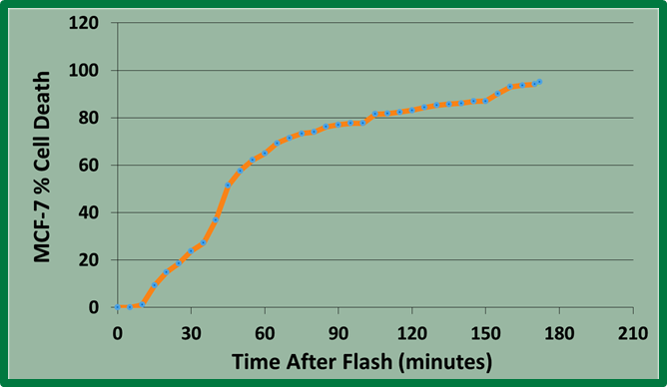
VNB optimized our LAIA technology into an UCNP that is activated by deep penetrating NIR light. The development of an upconverting light-activated nanoparticle allows for activation of intracellular acidosis by much deeper penetrating light than is currently used in other forms of light-activated cancer treatments. Following incubation with the UCNP, exposure of MCF-7 breast cancer cells to 980 nm near infrared light (400 mW, 10 minutes) resulted in 91.5% cancer cell death within 3 hours. Incorporation of LAIA in the form of an UCNP is important because it enables deeper penetration of the light to the tumor, thereby improving the clinical application of LAIA.
4. Actively targeting the UCNP to kill only prostate cancer:
VNB is currently improving compound delivery by actively targeting the NBA:Yt UCNP with PSMAb designed to detect PSMA expressed exclusively on the surface of prostate cancer cells for their first indication of prostate cancer. This PSMA-targeted UCNP, termed VNBp-1, will only enter cancer cells expressing PSMA on their surface. By targeting VNBp-1 to only prostate cancer cells expressing PSMA, VNB will effectively kill prostate cancer without damaging nearby healthy tissue. VNB plans to treat local prostate cancer as a first line treatment with VNBp-1, in combination with a trademarked 980 nm light source and trademarked endoscopic fiber optics. VNBp-1 will be used in conjunction with deep penetrating NIR light to elicit rapid intracellular acidosis and concomitant pH-induced apoptosis of the targeted prostate cancer cells. VNB has successfully formulated NBA:Yt UCNP’s with an average diameter of 40 nm, well below the maximum diameter of 150-200 nm for particles to undergo endocytosis. VNB’s nanoparticle delivery system has the potential to address a significant barrier currently facing the treatment of all tumors, specifically the ability to selectively kill the tumor without damaging adjacent healthy tissue. The ability to photo activate prostate cancer cells containing the targeted UCNP by deep penetrating light via the urethra and/or rectum provides a non-invasive treatment to kill only prostate cancer cells and spare nearby healthy tissue.
For development and optimization purposes only, in order to measure the effectiveness of our antibody/antigen binding and nanoparticle endocytosis, the PSMAb that is conjugated to our UCNP is labeled with the fluorescent marker, Alexafluor 488. When Alexafluor 488 is excited by 488 nm light, it will fluoresce, emitting green light. Using Alexafluor 488 conjugation to our PSMAb allows us to visualize the antibody and demonstrate the efficiency of the active targeting component to our technology. Recent data demonstrate that our targeted UCNP begins entering prostate cancer cells as early as five minutes after exposure and shows significant intracellular accumulation over a three-hour period.
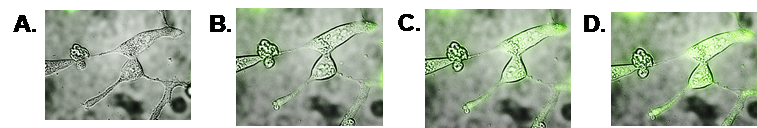
LNCaP prostate cancer cells following exposure to the PSMA-targeted UCNP. These sequential DIC images include the Alexafluor 488 fluorescence overlays, demonstrating the increases in intracellular compartmentalization of our PSMA-targeted UCNP. Panel A reflects one-minute following exposure of the cancer cells to the UCNP; Panels B, C, and D reflect green fluorescence after 90, 120, and 200 minutes of exposure, respectively.
5. Conducting proof of principle testing with VNBp-1 utilizing prostate cancer in vivo :
The optimal formulation of VNBp-1 and light delivery parameters will undergo a series of POP tests in vivo. The first POP test will involve nude mice bearing red-shifted firefly luciferase expression PSMA-positive LNCaP be exposed to the control condition of intratumoral injection of VNBp-1 only or the treatment condition consisting of intratumoral injection of VNBp-1 and external 980 nm photo activation. Additional POP testing will include an additional control condition using nude mice bearing GFP-expressing PSMA-negative PC-3-flu prostate cancer tumors. Analyses will include biodistribution of VNBp-1 to the tumor and nearby organs, quantification of tumor volume, tumor growth rate, survivability, hemocompatibility, and renal function. The culmination of this testing will deliver an active-targeted UCNP capable of selectively entering and killing only PSMA-expressing cancer cells in mice in response to deep-penetrating near infrared light.
Technology video: “How it Works”